Scientists find hydrogen cyanide, a key molecule for life formation, in Saturn’s icy moon Enceladus (Sci News)
- 18 Dec 2023
Why is it in the News?
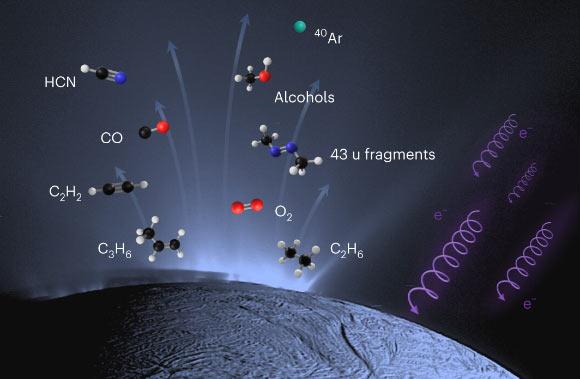
Recently, planetary scientists have detected several compounds of strong importance to the habitability of Saturn’s icy moon Enceladus, including hydrogen cyanide, acetylene, propylene and ethane, using data from NASA’s Cassini mission.
What is Enceladus?
- Enceladus is the sixth-largest icy moon of Saturn. It has a white, streaky surface made of water ice.
- Beneath this frozen crust lies a warmer, salty ocean that covers the whole moon.
- This circular moon is just about 500 km wide, with a surface temperature of -200°C.
- But its interiors host several sources of energy and heat.
- Enceladus is tugged and pulled in all directions by Saturn’s gravity and the gravity of other more massive moons, thus creating heat in its interior.
- Its rocky core may also be undergoing radioactive decay and chemical reactions that generate heat.
- Moreover, it is also an active source of water volcanism, where giant plumes of water, ice, dust, and gases are ejected into space like volcanic explosions.
- The material from the plumes replenishes one of the rings of Saturn as well, as the icy particles that make up the rings slowly drift inwards and get pulled into Saturn.
- The presence of a global saltwater ocean with nutrients and a heat source suggests a suitable aquatic environment for life.
- However, no life has been found on Enceladus or anywhere else beyond Earth.
About Hydrogen cyanide:
- Hydrogen cyanide is a chemical compound with the formula HCN and structural formula H−C≡N.
- It presents itself as a colourless or pale-blue liquid or gas, characterized by a bitter, almond-like fragrance.
- Alternatively referred to as hydrocyanic acid or HCN, this substance has the potential to disrupt the body's oxygen utilization, posing risks to the brain, heart, blood vessels, and lungs.
- While Hydrogen cyanide boasts excellent solvent properties for numerous salts, its utilization as a solvent is limited due to its inherent toxicity.
- In various industrial settings, it finds application in tasks such as fumigation, electroplating, mining, chemical synthesis, and the manufacturing of synthetic fibres, plastics, dyes, and pesticides.
