Foot-and-Mouth Disease (FMD)

- 01 Sep 2024
In News:
- Establishment of Disease-Free Zones:
- The Union government plans to create FMD-free zones in eight states: Karnataka, Tamil Nadu, Andhra Pradesh, Telangana, Uttarakhand, Punjab, Haryana, Maharashtra, and Gujarat.
- Aim: Expand export opportunities for Indian animal products and enhance global market presence.
- Vaccination Efforts:
- Advanced vaccination initiatives are underway in the identified states, as stated by Alka Upadhyaya, Secretary of the Department of Animal Husbandry and Dairying.
- Collaborative Workshop:
- A workshop on ‘Animal Infectious Disease Prioritisation’ was held in collaboration with the Food and Agriculture Organisation.
- Focus: Prioritized 20 major animal infectious diseases based on severity, transmissibility, and national importance.
- Action Plan:
- Formulated focusing on five critical areas:
- Coordination
- Communication
- Monitoring and surveillance
- Prevention and control
- Therapeutics and socio-economic planning
- Formulated focusing on five critical areas:
- Regional Disease Prioritization:
- Strengthening regional-level prioritization of animal diseases for tailored control strategies.
- Overview of FMD:
- Highly contagious viral disease affecting cloven-hoofed animals like cattle, swine, sheep, and goats, but not horses or cats.
- Significant economic impact due to its effect on livestock production and trade.
Key Characteristics of FMD:
- Transmission:
- Virus present in excretions and secretions; aerosolized virus can infect other animals via respiratory or oral routes.
- Symptoms:
- Fever, blister-like sores on the tongue, lips, and hooves.
- High mortality in young animals, with production losses noted even post-recovery.
- Vaccination:
- Available vaccines must be matched to specific virus types/subtypes.
Repairability Index for Mobile and Electronic Sectors

- 01 Sep 2024
In News:
- The Department of Consumer Affairs (DoCA), Government of India, has established a committee of experts to create a framework for the Repairability Index.
- Objective:
- Enhance consumer transparency regarding product repairability.
- Promote sustainable practices in the tech industry.
- National Workshop:
- Held on August 29, 2024, focusing on the Right to Repair in the Mobile and Electronics Sector.
- Aimed to gather industry stakeholders to agree on evaluating components for the Repairability Index.
- Key Goals:
- Address the rapid demand and short lifespan of mobile and electronic devices.
- Provide essential repair information and ensure access to spare parts, even for discontinued products.
- Repairability Index:
- A consumer-focused tool that helps in making informed product decisions based on repairability.
- Aims to standardize repairability assessments, enabling easier product comparisons.
- Consumer Empowerment:
- The index fosters mindful consumption and sustainability.
- Ensures affordable repair options and improves overall consumer satisfaction by addressing information gaps.
- Key components of the Repair Ecosystem:
- Comprehensive Repair Information: Access to repair manuals/DIYs, diagnostics, and a list of necessary tools and parts.
- Accessible Spare Parts: Easily identifiable and timely delivery of spare parts.
- Affordable Tools: Inexpensive, widely available, and safe tools for consumers.
- Modular Design: Key components designed for independent access and modularity.
- Economic Feasibility: Ensuring that the cost of repair parts and labor is affordable for consumers.
Taking into account the above necessities the committee is expected to recommend enabling framework for Policies/Rules/Guidelines which support repairability and integration of repairability index with the extant regulatory provisions in mobile and electronics sector to enhance consumer experiences in reusing the mobile and electronics products they own.
The committee will submit a comprehensive report including a framework for repairability index in Indian context by 15th November, 2024.
India's Biotech Revolution

- 01 Sep 2024
In News:
The Indian Cabinet has recently approved the BioE3 (Biotechnology for Economy, Environment, and Employment) proposal, a significant move to advance the country’s biotechnology sector.
Scheduled to take effect on April 1, 2025, the BioE3 policy aims to capitalize on India's biotechnology potential by focusing on six key areas: bio-based chemicals, functional foods, precision biotherapeutics, climate-resilient agriculture, carbon capture, and marine/space research.
Current Status of India’s Biotechnology Sector
India ranks among the top 12 biotechnology destinations globally and is the third-largest in the Asia-Pacific region. As of 2024, India's Bioeconomy is valued at an estimated USD 130 billion. The sector is integral to India’s goal of becoming a USD 5 trillion economy by 2024, with biotechnology contributing about 3% to the global market share.
Biotechnology Categories in India:
- Biopharmaceuticals: India is a major supplier of low-cost drugs and vaccines, leading in biosimilars with the highest number of approvals.
- Bio-Agriculture: India dedicates approximately 55% of its land to agriculture, holding the fifth-largest area of organic agricultural land worldwide. The sector's contribution to the Bioeconomy is expected to grow from USD 10.5 billion to USD 20 billion by 2025.
- Bio-Industrial: Biotechnology is enhancing industrial processes, manufacturing, and waste disposal.
- Bio IT & BioServices: India excels in contract manufacturing, research, and clinical trials, hosting the highest number of US FDA-approved plants outside the US.
Government Initiatives:
- 100% foreign direct investment (FDI) is permitted in greenfield pharma and medical devices.
- The National Biotechnology Development Strategy 2021-25 aims to make India a global leader in biotechnology, targeting a USD 150 billion Bioeconomy by 2025.
- The Department of Biotechnology has established 51 Biotech-KISAN hubs to connect farmers with scientific advancements.
- The Union Budget 2023-24 includes INR 10,000 crore for 500 ‘waste to wealth’ plants under the GOBARdhan scheme.
- The GenomeIndia Project focuses on sequencing and analyzing the Indian population’s genomes to aid public health.
Challenges and Recommendations
Challenges:
- Regulatory Hurdles: The complex approval process for GMOs and overlapping regulatory bodies slow down progress.
- Funding Issues: Limited funding and high risks deter investment. The biotechnology sector receives only 0.05% of India's GDP from the Central Government.
- Infrastructure Gaps: Inadequate research facilities and cold chain infrastructure hamper progress.
- IP Concerns: Intellectual property protection remains weak, affecting innovation.
- Global Competition: Indian firms face stiff competition from established global players.
- Talent Shortages: A brain drain and skills mismatch impede growth.
- Ethical Dilemmas: Ethical issues related to GMOs and gene editing pose challenges.
Recommendations:
- Regulatory Streamlining: Establish a unified Biotechnology Regulatory Authority and adopt a risk-based assessment approach.
- Innovative Funding: Create a Biotechnology Investment Fund with public-private partnerships.
- Talent Development: Launch skill development programs and integrate biotech training into various disciplines.
- Infrastructure Investment: Develop shared high-end research facilities and upgrade cold chain infrastructure.
- IP Strengthening: Enhance the IPR regime and establish a Biotech Patent Pool.
- Leverage Make in India: Expand the Production Linked Incentive (PLI) scheme to cover more biotech products and establish specialized manufacturing corridors.
Unified Pension Scheme

- 01 Sep 2024
In News:
The new Unified Pension Scheme (UPS), set to launch on April 1, 2025, aims to provide improved old age income security. Around 23 lakh Central government employees will benefit from this new scheme, and those currently under the National Pension System (NPS) will have the option to switch to UPS. States can also adopt the UPS for their employees, but they will need to secure funding from their own resources.
Key Components of UPS
The UPS introduces several enhancements to pension benefits:
- Pension Benefits: Employees will receive half of their average basic pay over the final 12 months of service as a monthly pension after completing a minimum of 25 years of service. For those with less than 25 years, the pension will be proportionately reduced, with a minimum pension of ?10,000 for those with at least 10 years of service.
- Family Pension: A family pension equivalent to 60% of the employee's pension will be provided to dependents upon the employee's death.
- Inflation Adjustment: Pension incomes will be adjusted in line with the consumer price trends for industrial workers, similar to the dearness relief provided to current government employees.
- Superannuation Payout: In addition to gratuity, a lumpsum superannuation payout will be given, amounting to 1/10th of the employee’s monthly emoluments for every six months of service.
Differences from the Current System
The new UPS combines features from the Old Pension Scheme (OPS) and NPS:
- Old Pension Scheme (OPS): Employees who joined before January 1, 2004, are covered under OPS, which guarantees a pension of 50% of the last drawn salary, adjusted for dearness allowance. It also offers a family pension of 60% of the last drawn pension, with provisions for commutation and additional increases for pensioners over 80 years of age.
- National Pension System (NPS): Introduced in 2004, NPS replaced OPS for new employees, shifting from a defined benefits system to a defined contribution scheme. Employees and the employer contribute a percentage of the salary to market-linked securities, with no guaranteed pension amount, only a corpus that must be used to buy an annuity upon retirement.
The UPS aims to blend the certainty of OPS with the funded approach of NPS. While employees' contributions will be capped at 10% of their salary, the government will contribute 18.5%, with potential adjustments over time. The government will cover any shortfall between investment returns and pension promises.
Reasons for the Change
The transition to UPS addresses concerns raised by government employees and political pressure regarding the NPS. Employees have criticized NPS for its lack of guaranteed pension benefits compared to OPS. The issue has become politically significant, with opposition parties promising to revert to OPS in various states.
In March 2023, Finance Minister Nirmala Sitharaman announced a review of NPS led by former Finance Secretary T.V. Somanathan. Though the review’s findings are yet to be made public, the introduction of UPS reflects a compromise balancing employee expectations with fiscal prudence.
Reactions and Future Impact
Central government employees generally welcome the UPS, recognizing it as a step toward addressing the shortcomings of NPS. However, there are concerns about the contributory nature of UPS and the absence of a commutation option like in OPS. Economists are analyzing the scheme's financial implications, with an expected additional cost of ?7,050 crore this year for the government. Future pension payouts may increase but are anticipated to be manageable with higher revenue growth.
The UPS marks a significant shift in pension policy, aiming to provide greater financial security for government employees while managing fiscal responsibilities.
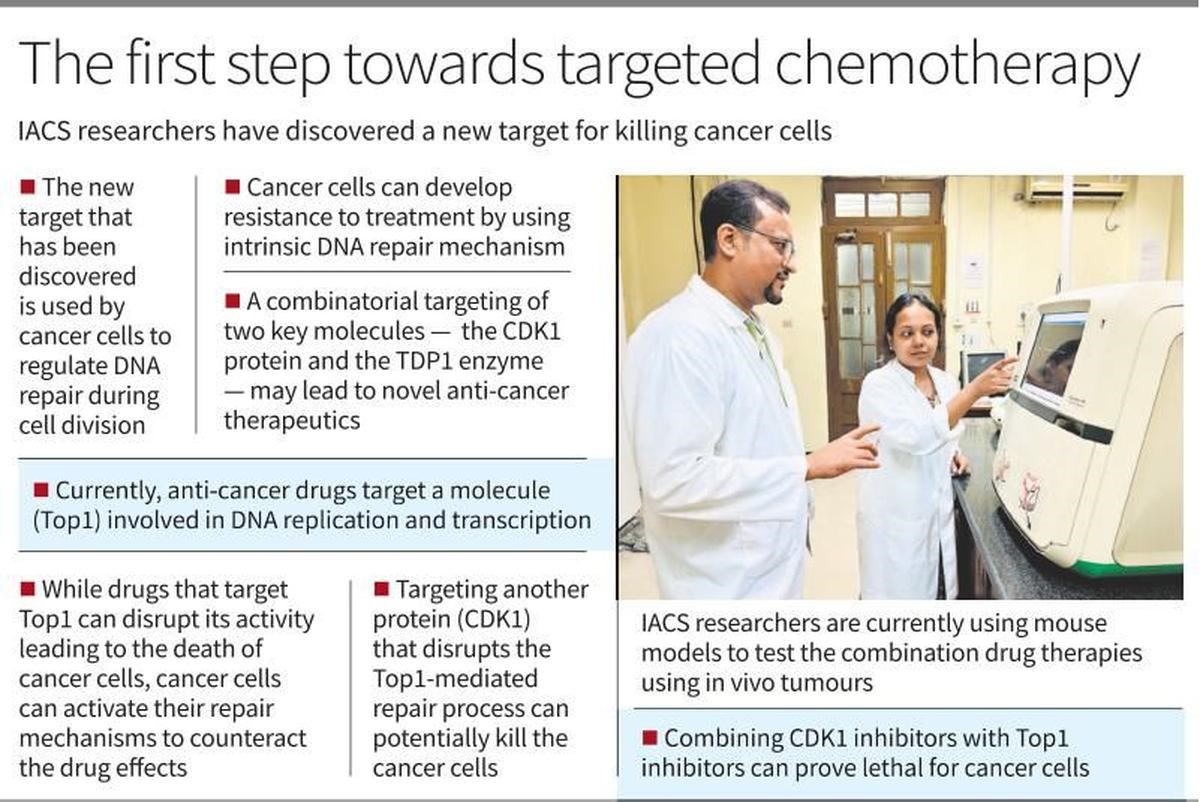
New Target for Cancer Treatment Discovered by IACS Scientists
- 01 Sep 2024
In News:
In a significant breakthrough, scientists at the Indian Association for the Cultivation of Science (IACS) in Kolkata have identified a new target for cancer therapy. Their study, recently published in The EMBO Journal, focuses on how cancer cells manage DNA repair during cell division, potentially paving the way for more effective treatments.
Key Findings
The researchers explored how cancer cells respond to topoisomerase 1 (Top1)-targeted chemotherapy. Top1 inhibitors, such as camptothecin, topotecan, and irinotecan, disrupt DNA replication and transcription, causing damage that usually leads to cell death. However, cancer cells can sometimes develop resistance by employing internal DNA repair mechanisms, primarily involving a protein called TDP1.
Mechanism of Action
Top1 is crucial for relaxing DNA supercoils during cell division, a process necessary for accurate chromosome segregation. Drugs targeting Top1 can kill cancer cells by preventing this relaxation. Nonetheless, cancer cells counteract this damage with TDP1, which repairs the DNA and promotes cell survival.
The IACS team discovered that TDP1's function is influenced by its phosphorylation status, which changes during the cell cycle and drug treatment. This modification helps TDP1 detach from chromosomes during cell division, a mechanism that helps cells survive despite the presence of chemotherapy drugs.
Novel Therapeutic Approach
The researchers propose a novel approach that combines inhibitors of two key molecules: CDK1 protein and TDP1 enzyme. CDK1 plays a critical role in regulating the cell cycle, while TDP1 is involved in repairing DNA damage. By inhibiting both, the researchers aim to disrupt the cancer cell's ability to repair DNA damage caused by Top1 inhibitors.
This combinatorial targeting strategy could enhance the effectiveness of cancer treatments. While Top1 inhibitors induce DNA damage, CDK1 inhibitors could prevent the repair of this damage or halt the cell cycle, making it difficult for cancer cells to survive. This dual-target approach may also help overcome resistance mechanisms that cancer cells develop against single-agent therapies.
Clinical Implications
CDK1 inhibitors, including avotaciclib, alvocidib, roniciclib, riviciclib, and dinaciclib, are currently in various stages of clinical trials. These drugs can be used alone or in combination with other DNA-damaging agents. Combining CDK1 inhibitors with Top1 inhibitors holds promise for significantly improving cancer treatment outcomes by targeting different aspects of the cell cycle and DNA replication.
Although the study was conducted using human breast cancer cells, the findings suggest potential benefits for patients with other types of cancer, such as ovarian, colorectal, and small cell lung cancers (SCLC). SCLC, in particular, is associated with tobacco smoking and could potentially benefit from this new combinatorial approach.
Conclusion
The IACS study opens new possibilities for cancer treatment by targeting DNA repair mechanisms in cancer cells. By combining CDK1 and Top1 inhibitors, the researchers aim to enhance the effectiveness of chemotherapy and overcome resistance. Further research, including clinical trials, will be essential to validate these findings and develop personalized cancer therapies that could improve patient outcomes across various cancer types.
