Haber-Bosch process
- 15 Oct 2024
In News:
The Haber-Bosch process has fundamentally transformed agricultural practices and global food production, enabling the conversion of atmospheric nitrogen into ammonia, which is essential for fertilizers.
The Nitrogen Molecule
- Composition: Nitrogen primarily exists as molecular nitrogen (N?) in the atmosphere, where two nitrogen atoms are bonded with a strong triple bond. This bond is very stable and requires significant energy (946 kJ/mol) to break, rendering N? largely inert and unavailable for direct use by plants.
Nitrogen in Nature
- Natural Fixation: In nature, the energy required to break the N? bond is typically provided by phenomena like lightning, which converts nitrogen to reactive forms such as nitrogen oxides (NO and NO?). These can subsequently form nitric acid when they react with water, depositing reactive nitrogen through rainfall.
- Microbial Processes: Certain bacteria, including Azotobacter and Rhizobia, can fix atmospheric nitrogen into reactive forms, supporting plant growth. Azolla, a fern with a symbiotic cyanobacterium, also helps in nitrogen fixation.
The Nitrogen Cycle
- Plant Uptake: Plants absorb reactive nitrogen in the form of ammonium (NH??) and nitrate (NO??) from the soil, essential for synthesizing proteins and other vital compounds. Humans and animals rely on plants for their nitrogen intake.
- Cycle Completeness: While nitrogen is returned to the soil through excretion and decomposition, some is lost back to the atmosphere as N?. This loss contributes to the depletion of soil nitrogen, especially in crops that do not fix their own nitrogen.
Ammonia Production
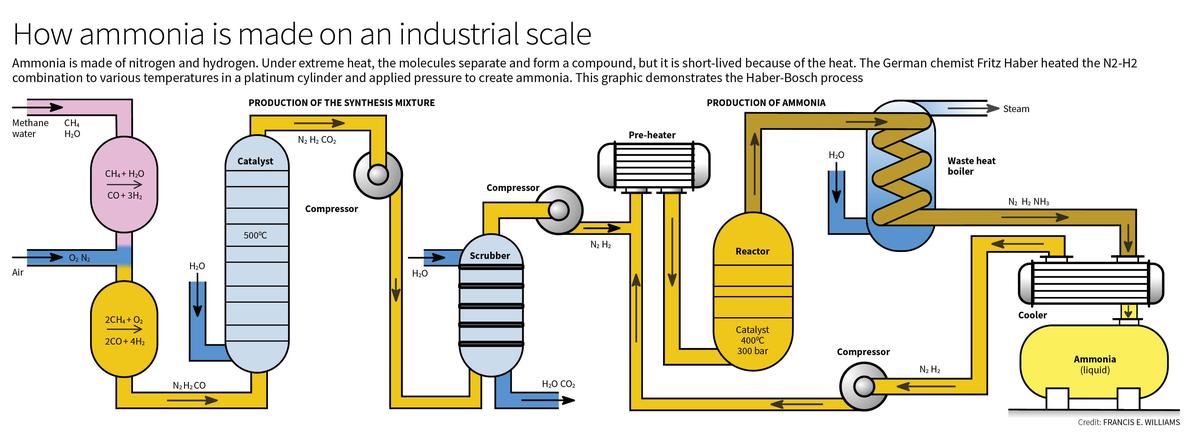
- Haber-Bosch Process: This process synthesizes ammonia from nitrogen and hydrogen under high pressure and temperature, using a catalyst to enhance efficiency. Initially developed by Fritz Haber and scaled by Carl Bosch, this method became the backbone of modern fertilizer production.
Benefits and Downsides of Fertilizers
- Food Security: The Haber-Bosch process has significantly increased food production, contributing to a remarkable rise in global food supply and preventing widespread hunger. It is estimated that one-third of the world’s population relies on fertilizers produced via this process for their food.
- Environmental Impact: The widespread use of nitrogen fertilizers can lead to environmental issues:
- Excess Nutrients: Over-application can result in nutrient runoff into water bodies, causing eutrophication, which depletes oxygen and harms aquatic life.
- Acid Rain: Reactive nitrogen can contribute to acid rain, affecting soil health and biodiversity.
- Soil Degradation: Continuous fertilizer use without adequate replenishment of nutrients can degrade soil quality over time.
While the Haber-Bosch process is crucial for modern agriculture and food security, it also presents significant environmental challenges. The balance between using fertilizers effectively and sustainably is essential to ensure that technological advancements do not come at the cost of ecological health. As such, addressing food security requires not just technological innovation, but also thoughtful political and social engagement to manage resources responsibly.
