Indian Scientists Develop Novel Gene Therapy for Haemophilia
- 12 Dec 2024
In News:
Indian scientists have developed a successful gene therapy treatment for severe haemophilia A, a rare inherited blood disorder causing spontaneous, potentially fatal bleeding episodes.
Key Highlights:
Trial Success:
- The trial, conducted at Christian Medical College (CMC), Vellore, involved five patients from Tamil Nadu.
- Results: None of the five patients reported bleeding episodes for over a year after receiving the treatment. The follow-up period averaged 14 months.
- This marks a significant improvement, as haemophilia patients typically experience frequent bleeding episodes requiring regular treatment.
Gene Therapy as a One-Time Solution:
- Traditional treatments involve frequent injections of clotting factors to prevent bleeding.
- The new gene therapy offers a one-time solution, teaching the body to produce enough clotting factor to prevent hemorrhages.
Haemophilia A - Overview:
- Caused by the absence of Factor VIII, a critical blood-clotting protein.
- Hemophilia A primarily affects males (since it's an X-linked disorder), though some females with two defective X chromosomes can also develop the condition.
- Symptoms include prolonged bleeding from minor injuries or internal bleeding in joints and muscles.
Current Treatment Challenges:
- Haemophilia treatments can be expensive and require lifelong care, costing up to ?2.54 crore over a 10-year period.
- The therapy requires repeated infusions of clotting factors or synthetic alternatives, which can be burdensome.
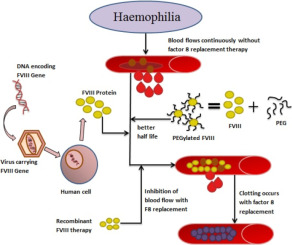
Gene Therapy Details:
- The gene therapy used in this trial involves fusing stem cells with the gene for Factor VIII using a lentivirus vector (safer than other vectors like adenovirus).
- This therapy eliminates the need for repeated Factor VIII infusions, providing a more cost-effective and sustainable solution.
Global Context:
- India has one of the world’s largest haemophilia populations, with an estimated 40,000 to 100,000 patients.
- The success of this gene therapy in India could lead to localized production, reducing treatment costs and increasing accessibility to gene therapy in resource-constrained settings.
Comparison with Roctavian:
- Roctavian, the only FDA-approved gene therapy for haemophilia A, also uses gene delivery to produce Factor VIII, but requires immunosuppressive therapy and is not approved for children.
- In contrast, the Vellore trial's lentivirus-based approach is considered safer, especially for children, with the potential for broader application.
