Large Ozone Hole Detected Over Antarctica (The Hindu)
- 13 Oct 2023
Why is it in the News?
As part of the EU’s environmental monitoring program, the European Space Agency Copernicus Sentinel-5P satellite detected a giant hole in the ozone layer over Antarctica recently.
What is an Ozone Hole?
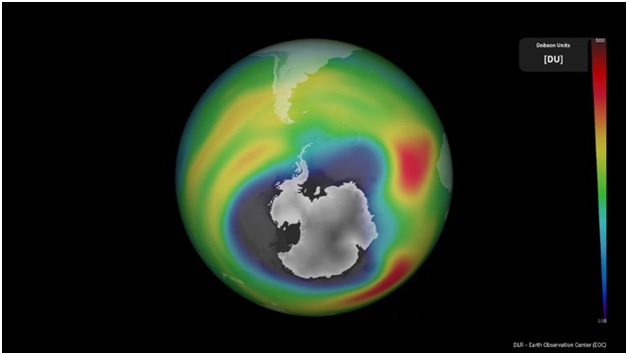
- An ozone hole refers to a region in the stratosphere above Antarctica where the ozone layer experiences significant depletion.
- Contrary to the term "hole," it doesn't imply a complete absence of ozone; rather, scientists use the term metaphorically to indicate an area where ozone concentrations fall below the historical threshold of 220 Dobson Units.
- The dimensions of the ozone hole over Antarctica fluctuate annually, typically forming in August and dissipating by November or December, driven by distinct climatic conditions in the region.
Mechanism Behind Ozone Hole Formation:
- The emergence of the ozone hole is linked to the Earth's rotation, creating specific winds over the enclosed Antarctic landmass.
- The polar vortex, characterized by robust winds around the poles, is a key factor in the dynamics of the ozone hole.
- During winter, the polar vortex forms due to temperature variations, acting as a barrier that separates polar air from warmer air at lower latitudes.
- This isolation fosters the formation of polar stratospheric clouds (PSCs), triggering reactions leading to ozone depletion.
- Chemical processes occurring on the surface of PSCs activate chlorine and bromine compounds, particularly chlorine, acting as catalysts in ozone-depleting reactions.
- When exposed to sunlight, these compounds catalyze the breakdown of ozone molecules.
- The size and intensity of the polar vortex directly influence the extent of ozone depletion.
- As the vortex weakens in spring, the mixing of polar air with warmer air from lower latitudes gradually closes the ozone hole, contributing to the restoration of the ozone layer.
What Caused the Giant Ozone Hole in 2023?
- Scientists believe this year’s big ozone hole could be due to the volcanic eruptions at Hunga Tongain Tonga during December 2022 and January 2023.
- In contrast to typical volcanic eruptions that release gases primarily within the lower atmosphere, this specific event ejected a substantial quantity of water vapour into the stratosphere.
- The presence of water vapour, along with other ozone-depleting elements such as bromine and iodine, had repercussions on the ozone layer through chemical reactions, ultimately modifying its heating rate.
Human-caused Ozone Holes:
- While this year’s Antarctic ozone hole was likely due to a volcanic eruption, scientists became aware that human activities were creating huge ozone holes in the 1970s.
- Ground and satellite-based measurements detected the holes, which were caused by the widespread use of chemicals called chlorofluorocarbons.
- “The culprit behind ozone depletion was not aerosols in aerosol cans, but the propellants we use as gases to propel the solutions inside.
- These gaseous propellants contain chlorine, which is released high in the stratosphere and depletes the ozone.
- The world took action after scientists raised alarm over the ozone holes, and quickly.
- In 1987, The Montreal Protocol was created to protect the ozone layer by phasing out the production of these harmful substances.
- The protocol was effective as ozone holes got smaller in the decades after ozone-depleting gas emissions were controlled.
Is there any Relation Between Climate Change and Ozone Holes?
- Ozone depletion is not considered a primary catalyst for global climate change.
- Nevertheless, there are indications that the rising global temperatures associated with climate change may impact the dynamics of ozone holes.
- Recent instances of substantial ozone holes have been associated with climate change, particularly the escalation of wildfires.
- The heightened frequency and intensity of wildfires, often exacerbated by climate change, release more smoke into the stratosphere, potentially contributing to additional ozone depletion.
- While ozone holes may yield a cooling effect by diminishing the greenhouse gas impact (as the loss of ozone allows slightly more heat to escape into space from that region), they can also disrupt the normal progression of seasons, leading to prolonged wintertime conditions.
What is Ozone (O3)?
- Formation: Ozone molecules result from the interaction of ultraviolet (UV) radiation from the Sun with O2 molecules.
- During this process, an O2 molecule is split, and the two liberated oxygen atoms combine with other O2 molecules, forming O3 molecules.
- While oxygen (O2) constitutes 21% of Earth's atmosphere, ozone makes up less than 0.001%.
- Ozone Layer: The ozone layer, crucial for life on Earth, is primarily generated in the stratosphere where UV radiation is more intense due to thinner air at higher altitudes.
- This region, known as the 'ozone layer,' spans approximately 10 to 40 km in altitude, with a peak of around 25 km.
- Significance of Ozone Layer: The ozone layer plays a vital role in protecting life on Earth by absorbing the most harmful type of UV radiation, UV-B radiation, with wavelengths between 280 and 315 nanometres.
- Shielding against UV rays is significant in reducing skin cancer rates, as most skin cancers are linked to excessive UV radiation exposure.
- As UV radiation is absorbed by ozone in the stratosphere, it leads to the heating of the surrounding air, creating the stratospheric temperature inversion.
- Measurement of Stratospheric Ozone: Stratospheric ozone is quantified as the total amount present in an atmospheric column in Dobson units.
- One Dobson unit represents the amount of ozone that would form a layer 0.01 mm thick at average sea-level pressure and temperature.
- Ozone Production: Most stratospheric ozone is produced at tropical latitudes, and high-altitude winds disperse it globally.
- Ozone undergoes continual formation and breakdown, with its distribution across the planet being neither uniform nor constant.
- Natural processes historically balanced ozone formation and breakdown, but human activities in recent decades have accelerated ozone depletion.
- Ground-level ozone formation contributes to 'photochemical smog,' posing a health hazard when ozone levels become elevated due to its toxic nature.
