CAR-T Cell Therapy (Indian Express)
- 03 Nov 2023
Why is it in the News?
The drugs regulator has granted market authorisation to India's breakthrough CAR-T cell therapy for patients with B-cell lymphomas who didn't respond to standard treatments like chemotherapy.
News Summary:
- India’s first indigenously developed Chimeric Antigen Receptor (CAR)-T Cell T therapy, a cutting-edge treatment for specific types of cancer patients, has shown promising results and could be the safest therapy in this category so far, researchers have said.
- The therapy was tested on six paediatric patients of Acute Lymphocytic Leukaemia and 10 adults suffering from B-cell lymphoma as part of phase one clinical trials by researchers associated with the Tata Memorial Hospital in Mumbai and the Indian Institute of Technology, Bombay.
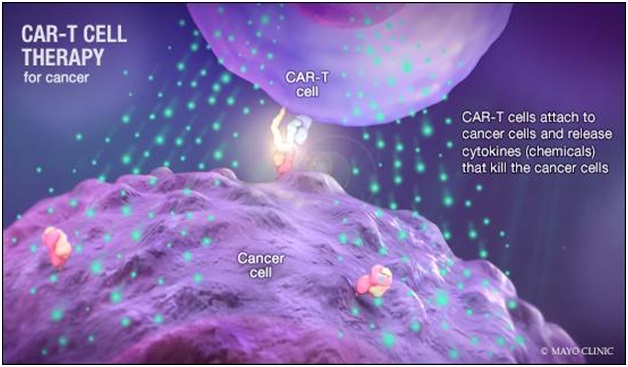
What is CAR-T cell therapy, and how do CAR-T cells find and destroy cancer cells?
- CAR-T is a revolutionary therapy that modifies immune cells, specifically T-cells, by turning them into potent cancer fighters known as CAR-T cells.
- T-cells are special cells (white blood cells that find and fight illness and infection) whose primary function is cytotoxic, meaning they can kill other cells.
- In CAR-T therapy, we genetically modify them into cancer-fighting cells. These supercharged cells are then put back into the body, and they go after cancer cells — especially in blood cancers like leukaemia and lymphomas.
Here's how CAR-T cell therapy works?
- Collection: First, a patient's T cells are taken from their blood through a process called "leukapheresis."
- Genetic Modification: These T cells are then sent to a laboratory where they are genetically modified to make them better at attacking cancer.
- Preparation: Before the modified T cells are returned to the patient, the patient typically undergoes several days of chemotherapy.
- This chemotherapy helps create the right environment in the body for the modified T cells to work against the cancer.
- Infusion: Once the environment is ready, the patient receives an infusion of the modified T cells.
- This process is similar to receiving a blood transfusion and takes about 30 to 90 minutes.
- Activation: The CAR-T cells become active and start to attack the cancer cells.
- However, this activation process can lead to side effects, such as a high fever, a fast heart rate, low blood pressure, and low blood oxygen levels.
- Some patients may also experience temporary neurological effects like confusion, tremors, and difficulty communicating.
- Recovery typically occurs within two weeks after one cycle of the treatment.
- Approximately 70% of patients respond to the treatment, with variations between leukaemia and lymphoma cases.
- About 50% of these responsive patients achieve a complete response.
How effective and different is this from other cancer treatments like, say, chemotherapy?
- While chemotherapy and immunotherapy may add a few months or years to a cancer patient’s life, cell-and-gene therapy is designed to cure and provide lifelong benefits.
- It makes treatment easier with a one-time therapy [unlike several sessions of chemotherapy] that can be truly transformative [for a patient].
- It’s a lifeline for non-responsive cancer patients.
What is NexCAR19?
- NexCar19 is a type of CAR-T and gene therapy developed indigenously in India by ImmunoACT, which is a company incubated at IIT Bombay.
- This therapy is designed to target cancer cells that carry the CD19 protein.
- This protein acts like a flag on cancer cells, which allows CAR-T cells to recognise and attach themselves to the cancer cells and start the process of elimination.
- India is now one of the first developing countries to have its indigenous CAR-T and gene therapy platform.
- Even some developed nations don’t have their own CAR-T therapies; they import them from the United States or Europe.
Who is eligible for the NexCAR19 therapy?
- This therapy is for people with B-cell lymphomas who didn’t respond to standard treatments like chemotherapy, leading to relapse or recurrence of the cancer.
- B-cell lymphoma is a kind of cancer that begins in white blood cells called lymphocytes.
- Lymphocytes produce antibodies, which are proteins that aid in the fight against infections.
- They are frequently discovered in lymph nodes and other lymphoid organs, like the spleen.
Are the children eligible for this therapy too?
- B-cell leukaemia is most common among children.
- The paediatric trial phase is currently underway at the Tata Memorial Hospital, in collaboration with IIT-Bombay.
- Although the therapy for children will not be any different, for now, ImmunoACT has received CDSCO approval for use in patients aged 15 years and older.
Is there any side-effects of this therapy?
- It significantly reduces drug-related toxicities, causing minimal harm to neurons and the central nervous system—a condition known as neurotoxicity.
- Neurotoxicity can occasionally occur when CAR-T cells recognize the CD19 protein and access the brain, potentially leading to life-threatening situations.
- Additionally, the therapy results in minimal Cytokine Release Syndrome (CRS), characterized by inflammation and hyperinflammation in the body due to the death of a substantial number of tumor cells.
Conclusion
The treatment is not intended for someone who is newly diagnosed. Instead, it’s an alternative for those who already have gone through other more conventional cancer therapy, such as chemotherapy or immunotherapy, and that treatment has been unsuccessful.
A considerable amount of research is underway to see if CAR-T cell therapy could be appropriate as a treatment for different forms of cancer, too, including other blood cancers such as multiple myeloma. So if a patient has a type of cancer that hasn’t responded well to traditional treatment, it may be worthwhile to explore the possibility of enrolling in a clinical research trial that’s using CAR-T cell therapy.
